How is chlorine made?
Chlorine is made in a process known as electrolysis
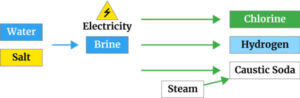
which also produces sodium hydroxide (caustic soda) and hydrogen as co-products. Electrolysis is a chemical process which uses electricity to split salt and water into chlorine, caustic and hydrogen. This takes place in an electrolyser. An electrolyser is made up of a positive electrode (or anode) and a negative electrode (or cathode). These electrodes are sat in salt water with the two electrodes being separated by a semi-permeable ‘barrier’ (also known as a membrane or a diaphragm). This barrier keeps the chlorine safely separate from the caustic and hydrogen.
Europe makes around 8 million tonnes of chlorine each year and you can find out more about European production of chlor-alkali here