How is caustic soda made?
Caustic soda is made in a process known as electrolysis
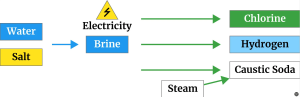
Which also produces chlorine and hydrogen as co-products. Electrolysis is a chemical process which uses electricity to split salt and water into chlorine, caustic and hydrogen. This takes place in an electrolyser. An electrolyser is made up of a positive electrode (or anode) and a negative electrode (or cathode). These electrodes are sat in salt water with the two electrodes being separated by a semi-permeable ‘barrier’ (also known as a membrane or a diaphragm). This barrier keeps the caustic soda safely separate from the chlorine.
Europe makes around 8 million tonnes of caustic soda each year and you can find out more about European production of Chlor-Alkali here.